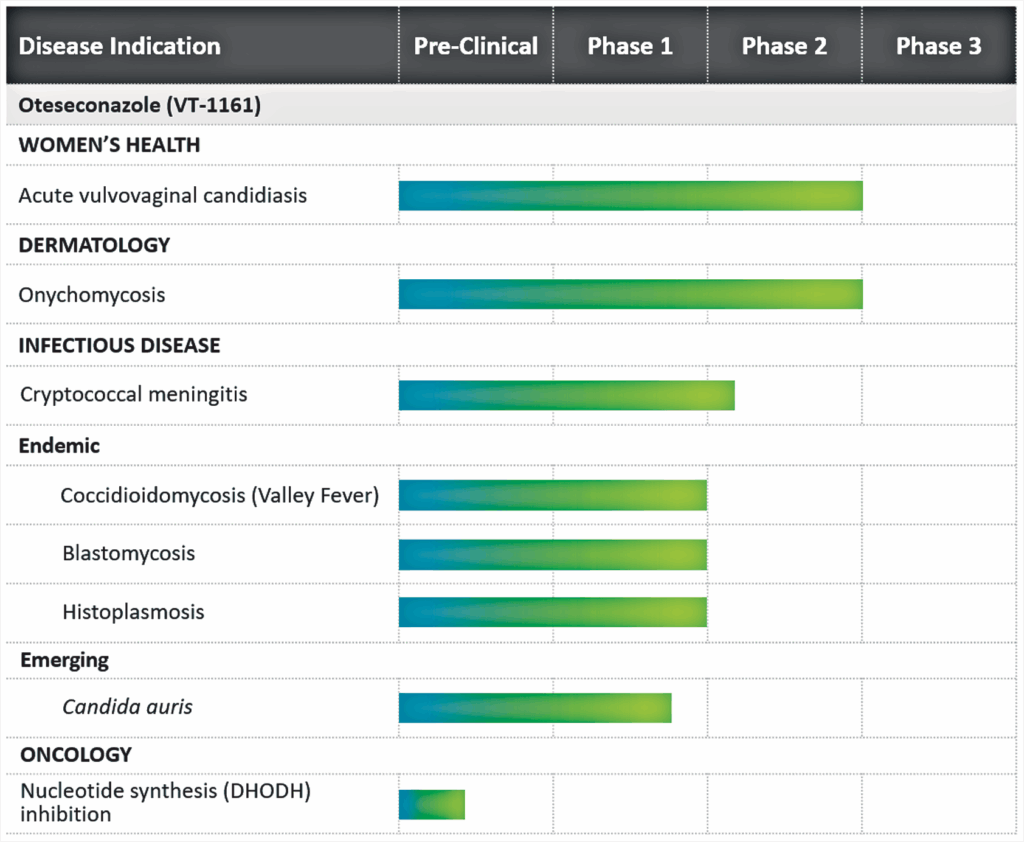
Our Pipeline
Mycovia is committed to the development of a robust pipeline of novel therapies to address the needs of patients living with overlooked medical conditions.
view our completed worldwide clinical trials for oteseconazole Clinical Trial Results

external research collaborations
We are actively seeking like-minded partners to collaborate in the development of innovative antifungal treatments. By working together, we can accelerate progress and drive advancements in the fight against fungal diseases.
We invite you to join us in reimagining the future of fungal disease treatment. We define collaborative research as a strategic partnership where multiple entities combine their financial resources, expertise, and technological capabilities to accelerate discovery, develop novel therapies, and solve complex healthcare challenges. Our research partners may include:
- Academic institutions
- Governmental groups
- Co-operative groups
- Pharmaceutical/ biotechnology
- Non-industry partners
To initiate a partnership today contact us here.
Investigator Initiated Research
This section is for individual healthcare professionals seeking to conduct an Investigator Initiated Study.
- Mycovia supports independent and ethical research aimed at augmenting the knowledge and experience of physicians and other healthcare providers and to facilitate advances in the prevention, diagnosis, and treatment of disease.
- Research concepts must be independently conceived. Mycovia’s involvement will be limited with no influence on study design, conduct of the research or interpretation of results.
- Investigators are responsible for all aspects of the research, including compliance with all regulatory requirements and fulfillment of investigator and sponsor responsibilities under applicable law.
Mycovia is currently accepting concept proposals for Investigator Initiated Research. To submit a proposal for consideration, please download and complete the form below and email it to [email protected].




